Forschung
Chalcogenometalates are excellent ligands for transition metals. Especially tetrathiometalates of Mo and W were used to synthesize heterobimetal clusters. Considering the importance of heterobimetal clusters in bioinorganic chemistry, catalysis, and medicine, it is not surprising that a lot of work has been done in this area. Many new V-M-S clusters (M = Fe, Cu, Ag, Au) were obtained, but the coordination chemistry of chalcogenoniobates and -tantalates was only examined in the last few years. A number of Nb/Ta-M-Q clusters containing M = Cu, Ag were synthesized, but only a few with M = Fe, Co, Ni. To us it is interesting to examine the structures and properties of new clusters of that kind and find out similarities and differences compared to known species.
In some cases it is a big challenge to explain the charge distribution, the binding situation, the magnetic behaviour, the redox properties and the reaction abilities of these new compounds. If you know the structure of a compound that contains several metal atoms you can usually find a number of reasonable ways to explain the charge distribution and the related bonding situation or magnetic behaviour. Therefore it is necessary to use different analysing methods and also theoretical approaches to come up with a plausible explanation.
The following picture represents one of the new compounds. It looks fairly simple. But it does not possess a trivial bonding situation and a lot of different measurements have to be done to fully understand the bonding situation, magnetic behaviour, redox properties, etc.
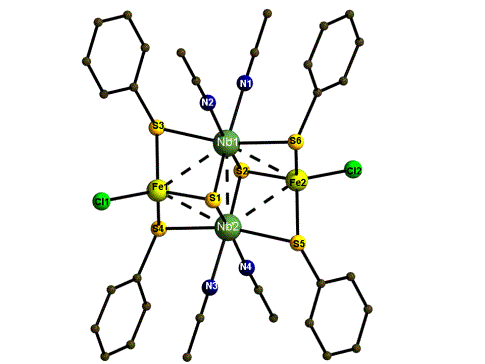
Abb.1 [Nb2Fe6Q2(QPh)4X2(MeCN)4] (Q = S, X = Cl 5; Q = Se, X = SePh 6)
The described field of study represents the possibility to not only get trained in synthesis, but also in different analysing techniques and the following interpretation of the collected results. It is therefore a challenging and diversified job to work on this topic.
