Inorganic-organic Hybrid Nanomaterials
Hybrid nanomaterials combine “the best” of two worlds - inorganic cations and organic anions. Both constituents have one or more specific functions. Thus, the inorganic cation provides a low solubility of the hybride. This is necessary in order to control particle nucleation and growth - and thus, to prepare nanoparticles. Moreover, the inorganic cation can be relevant for detection based on its eventual magnetism or based on X-ray absorption. The organic anion provides a certain function as well, for instance, fluorescence of gas sorption. In sum, hybrid nanomaterials allow for wide adaptability of composition and function. As the synthesis is typically conducted in water, access of nanoscale hybrids is most simple and of low cost. In the following, two types of nanoscale inorganic-organic hybrids are described that can be used as fluorescent markers in medicine (Figure 1) or for gas sorption and separation (Figure 2).
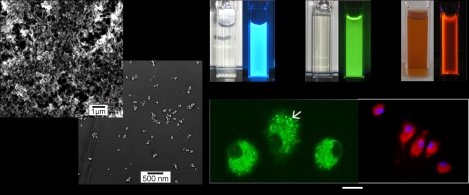
Figure 1: Particle size and emission (in suspension and in cells) of inorganic-organic hybrid nanoparticles such as [ZrO][(FMN)] (FMN: flavinmononucleotide).
The inorganic-organic hybrid [ZrO][(FMN)] (FMN: flavinmononucleotide) represents a new class of fluorecent materials showing bright green light emission. The nanomaterial comprises several important features, including quick and easy water-based synthesis, potentially low costs of production, a high biocompatibility, and a variable concentration of the incorporated dye, allowing for quasi-infinite number of luminescent centers. Typical key issues for quantum dots (e.g. CdSe) as well as metal-doped nanoparticles (e.g. LaPO4:Ce,Tb), such as high-temperature crystallization and core-shell type surface conditioning, do not need any consideration. Taking these aspects together, [ZrO][(FMN)] is a promising alternative to existing luminescent nanomaterials. Its use as a luminescent biomarker and its biocompatibility have been successfully tested as a proof of the concept in living mice and cells (Figure 12). Besides [ZrO][(FMN)] and the emission of green light, the concept of material can be extended to blue, red and infrared emission upon introducing other fluorescent dye anions as FMN.
The inorganic-organic hybrid magnesium aminoethyl phosphonate (Mg(AEP)(H2O) (particle diameter: 20 nm; specific surface: 322(10) m2g-1; pore volume: 0.9(1) cm3g-1) shows reversible CO2 sorption (152(5) mg g-1) at high pressure (≤110 bar). Mg(AEP)(H2O) represents a new material and composition as well as one of the first nanomaterials showing high CO2 uptake (Figure 13). In contrast to CO2 sorption, N2 uptake remains below 1.0(0.1) mg g-1. Thus, the inorganic-organic hybrid material exhibits an excellent CO2:N2 selectivity (~100) at high pressure (³20 bar). Based on enhanced selectivity, facile preparation, low-cost and less harmful constituents (i.e., Mg2+, [AEP]2-), Mg(AEP)(H2O) widens the range of materials and operation modes for CO2 capture.
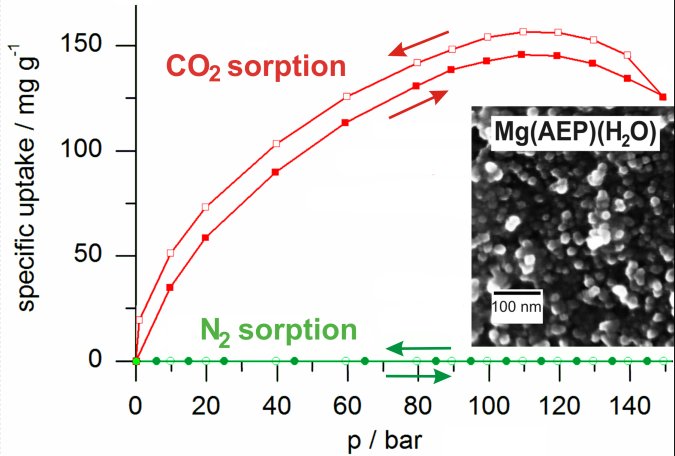
Figure 2:
CO2 and N2 sorption isotherms of Mg(AEP)(H2O) hybrid
nanoparticles (at 353 K).
For
more information see:
M. Roming, H.
Lünsdorf, K. E. J. Dittmar, C. Feldmann*, ZrO(HPO4)1-x(FMN)x:
Quick and Easy Synthesis of a Nanoscale Luminescent Biomarker, Angew. Chem. Int. Ed. 2010, 49, 632−637.
H. Goesmann, C. Feldmann*, Nanoparticulate Functional Materials (Review), Angew. Chem. Int. Ed. 2010,
49, 1362−1395.
P. Leidinger, S. Simonato, C. Feldmann*, Magnesium Aminoethyl Phosphonate (Mg(AEP)(H2O)):
An Inorganic-organic Hybrid Nanomaterial with High CO2:N2
Sorption Selectivity, Chem. Commun.
2012, 48, 7046–7048.